SPARC
Associated with Diabetes Complications, Cardiovascular Disease and Tumors
Alternative name: secreted protein acidic cysteine-rich (SPARC); Osteonectin; Basement-membrane protein 40; BM40 Secreted protein acidic and rich in cysteine/osteonectin/BM40, or SPARC, is a matrix-associated protein that elicits changes in cell shape, inhibits cell-cycle progression, and influences the synthesis of extracellular matrix (ECM).
SPARC regulates cell growth through interactions with the extracellular matrix and cytokines. SPARC binds calcium and copper, several types of collagen, albumin, thrombospondin, PDGF and cell membranes. There are two calcium binding sites; an acidic domain that binds 5 to 8 Ca(2+) with a low affinity and an EF-hand loop that binds a Ca(2+) ion with a high affinity.
|
Human
SPARC ELISA Kit SK00766-06 was used by Dr. Lee SH on following paper: Associations among
SPARC mRNA expression in adipose tissue, serum SPARC concentration and metabolicparameters
in Korean women
Objective:
Secreted protein acidic and rich in cysteine (SPARC) is expressed in most
tissues and is also secreted by adipocytes. The associations of SPARC mRNA
expression in visceral adipose tissue (VAT), subcutaneous abdominal adipose
tissue (SAT), serum SPARC concentration, and metabolic parameters in Korean
women are investigated.
Design
and Methods: This is a cross-sectional study.
Fifty-eight women were recruited, of whom 15 women who underwent bariatric
surgery for morbid obesity (BMI mean ± SD: 40.2±5.7 kg/m2), 16 who underwent
metabolic surgery for type 2 diabetes (BMI: 28.9±4.5 kg/m2), and, as a
control group, 27 who underwent gynecological surgery (BMI: 22.7±2.4 kg/m2).
Anthropometric variables, metabolic parameters, SPARC mRNA expression in
adipose tissue, and serum SPARC concentration were measured.
Results:
In all subjects, SPARC mRNA expression was significantly higher in SAT than
in VAT. Serum SPARC concentrations (mean ± SE) in morbidly obese subjects,
subjects with type 2 diabetes, and normal weight subjects were 267.3±40.2
ng/mL, 130.4±33.0 ng/mL, and 53.1±2.8 ng/mL, respectively. SPARC mRNA in SAT
was significantly correlated with BMI, whereas SPARC mRNA in VAT was
significantly correlated with BMI and VAT area. Serum SPARC concentration was
significantly correlated with BMI, waist circumference, total adipose tissue area,
and SAT area. After BMI adjustment, serum SPARC concentration was
significantly correlated with fasting insulin concentration and HOMA-IR
score. Multivariate regression analysis showed that BMI and HOMA-IR were
independently associated with serum SPARC concentration.
Conclusions:
Serum SPARC concentration is significantly correlated with obesity indices
and might be influenced by insulin resistance. These findings suggest that
SPARC may contribute to the metabolic dysregulation associated with obesity in
humans.
Lee SH et al. Obesity (Silver Spring). 2013
Nov;21(11):2296-302. doi: 10.1002/oby.20183. Epub 2013 May 13.
|
Inactivation of SPARC enhances high-fat diet-induced obesity in
mice
Secreted
protein, acidic and rich in cysteine (SPARC), a matricellular protein,
modulates extracellular matrix assembly and turnover in many physiological
processes. SPARC-null mice exhibit an increased accumulation of adipose tissue.
To distinguish between the functions of SPARC in adipogenesis during
development and adulthood, we studied wild-type (WT) and SPARC-null mice
maintained on a normal (low-fat) or high-fat (HF) diet. On an HF diet,
SPARC-null mice exhibited significantly greater weight gain, in comparison to
their WT counterparts, and had an enhanced cortical bone area that was likely
due to increased mechanical loading. Diet-induced obesity (DIO) was also
associated with an increase in vertebral trabecular bone in WT mice, but a
significant change in this parameter was not observed in SPARC-null animals. We
show that SPARC inhibits mitotic clonal expansion of preadipocytes at an early
stage of adipogenesis. Moreover, there were substantially diminished levels of
type I collagen in SPARC-null adipose tissue, as well as a reduction in the
number of cross-linked, mature collagen fibers. In the absence of SPARC, mice
show enhanced DIO. In adult animals, SPARC functions in the production and
remodeling of adipose tissue, as well as in the regulation of preadipocyte
differentiation.
Nie J., et al. Connect Tissue Res. 2011 Apr;52(2):99-108. Epub 2010 Jul
8.
|
SPARC: a key player in the pathologies
associated with obesity and diabetes.
SPARC (secreted protein acidic and rich in cysteine, also known as
osteonectin or BM-40) is a widely expressed profibrotic protein with pleiotropic
roles, which have been studied in a variety of conditions. Notably, SPARC is
linked to human obesity; SPARC derived from adipose tissue is associated with
insulin resistance and secretion of SPARC by adipose tissue is increased by
insulin and the adipokine leptin. Furthermore, SPARC is associated with
diabetes complications such as diabetic retinopathy and nephropathy,
conditions that are ameliorated in the Sparc-knockout mouse model. As a
regulator of the extracellular matrix, SPARC also contributes to
adipose-tissue fibrosis. Evidence suggests that adipose tissue becomes
increasingly fibrotic in obesity. Fibrosis of subcutaneous adipose tissue may
restrict accumulation of triglycerides in this type of tissue. These
triglycerides are, therefore, diverted and deposited as ectopic lipids in
other tissues such as the liver or as intramyocellular lipids in skeletal
muscle, which predisposes to insulin resistance. Hence, SPARC may represent a
novel and important link between obesity and diabetes mellitus. This Review
is focused on whether SPARC could be a key player in the pathology of obesity
and its related metabolic complications.
Kos K, Wilding JP.Nat Rev Endocrinol. 2010
Apr;6(4):225-35. Epub 2010 Mar 2. | Cardiac
extracellular matrix remodeling: fibrillar collagens and Secreted Protein
Acidic and Rich in Cysteine (SPARC)
The
cardiac interstitium is a unique and adaptable extracellular matrix (ECM) that
provides a milieu in which myocytes, fibroblasts, and endothelial cells
communicate and function. The composition of the ECM in the heart includes
structural proteins such as fibrillar collagens and matricellular proteins that
modulate cell:ECM interaction. Secreted Protein Acidic and Rich in Cysteine
(SPARC), a collagen-binding matricellular protein, serves a key role in
collagen assembly into the ECM. Recent results demonstrated increased cardiac
rupture, dysfunction and mortality in SPARC-null mice in response to myocardial
infarction that was associated with a decreased capacity to generate organized,
mature collagen fibers. In response to pressure overload induced-hypertrophy,
the decrease in insoluble collagen incorporation in the left ventricle of
SPARC-null hearts was coincident with diminished ventricular stiffness in
comparison to WT mice with pressure overload. This review will focus on the
role of SPARC in the regulation of interstitial collagen during cardiac
remodeling following myocardial infarction and pressure overload with a
discussion of potential cellular mechanisms that control SPARC-dependent
collagen assembly in the heart.
McCurdy S, et al. J Mol Cell Cardiol. 2010 Mar;48(3):544-9. Epub 2009 Jul
3.
|
Proteins involved in platelet signaling
are differentially regulated in acute coronary syndrome: a proteomic study
BACKGROUND: Platelets play a fundamental role in pathological events underlying
acute coronary syndrome (ACS). Because platelets do not have a nucleus,
proteomics constitutes an optimal approach to follow platelet molecular
events associated with the onset of the acute episode.
METHODOLOGY/PRINCIPAL
FINDINGS: We performed the first high-resolution two-dimensional gel
electrophoresis-based proteome analysis of circulating platelets from
patients with non-ST segment elevation ACS (NSTE-ACS). Proteins were identified
by mass spectrometry and validations were by western blotting. Forty protein
features (corresponding to 22 unique genes) were found to be differentially
regulated between NSTE-ACS patients and matched controls with chronic
ischemic cardiopathy. The number of differences decreased at day 5 (28) and 6
months after the acute event (5). Interestingly, a systems biology approach
demonstrated that 16 of the 22 differentially regulated proteins identified
are interconnected as part of a common network related to cell assembly and
organization and cell morphology, processes very related to platelet
activation. Indeed, 14 of those proteins are either signaling or
cytoskeletal, and nine of them are known to participate in platelet
activation by αIIbβ3 and/or GPVI receptors. Several of the proteins
identified participate in platelet activation through post-translational
modifications, as shown here for ILK, Src and Talin. Interestingly, the
platelet-secreted glycoprotein SPARC was down-regulated in NSTE-ACS patients
compared to stable controls, which is consistent with a secretion process
from activated platelets.
CONCLUSIONS/SIGNIFICANCE: The present study provides novel information on platelet proteome
changes associated with platelet activation in NSTE-ACS, highlighting the
presence of proteins involved in platelet signaling. This investigation paves
the way for future studies in the search for novel platelet-related
biomarkers and drug targets in ACS.
Fernández
Parguiña A, et al. PLoS One. 2010 Oct 14;5(10):e13404.
|
|
Identification of Adipocyte Genes
Regulated by Caloric Intake
Context:
Changes in energy intake have marked and rapid effects on metabolic
functions, and some of these effects may be due to changes in adipocyte gene
expression that precede alterations in body weight.
Objective:
The aim of the study was to identify adipocyte genes regulated by changes in
caloric intake independent of alterations in body weight.
Research
Design and Methods: Obese subjects given a very low-caloric
diet followed by gradual reintroduction of ordinary food and healthy subjects
subjected to overfeeding were investigated. Adipose tissue biopsies were
taken at multiple time-points, and gene expression was measured by DNA
microarray. Genes regulated in the obese subjects undergoing caloric
restriction followed by refeeding were identified using two-way ANOVA
corrected with Bonferroni. From these, genes regulated by caloric restriction
and oppositely during the weight-stable refeeding phase were identified in
the obese subjects. The genes that were also regulated, in the same direction
as the refeeding phase, in the healthy subjects after overfeeding were
defined as being regulated by caloric intake. Results were confirmed using
real-time PCR or immunoassay.
Results:
Using a significance level of P < 0.05 for all comparisons, 52 genes were
down-regulated, and 50 were up-regulated by caloric restriction and regulated
in the opposite direction by refeeding and overfeeding. Among these were
genes involved in lipogenesis (ACLY, ACACA, FASN, SCD), control of protein
synthesis (4EBP1, 4EBP2), {beta}-oxidation (CPT1B), and insulin resistance
(PEDF, SPARC).
Conclusions:
Metabolic genes involved in lipogenesis, protein synthesis, and insulin
resistance are central in the transcriptional response of adipocytes to
changes in caloric intake.
Niclas Franck et al. Journal of Clinical Endocrinology &
Metabolism , doi:10.1210/jc.2009-2534. published online onNovember 3, 2010
|
|

|

|
|
|
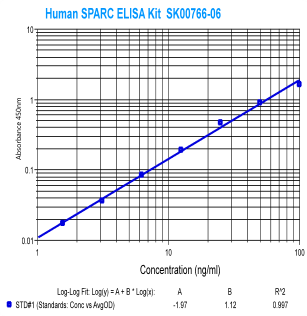
Human
SPARC (Osteonectin) ELISA
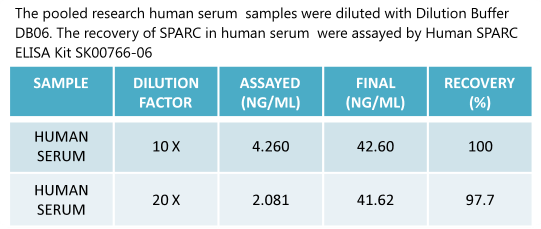
Code No.: SK00766-06
Size: 96 T
Standard Range:1.56-100 ng/ml
Sensitivity:0.5 ng/ml
Sample Type: serum, EDTA plasma
Dilution Factor: 40
IntraCV: 6-8%
InterCV: 10-12%
Protocol: PDF
|
|
|
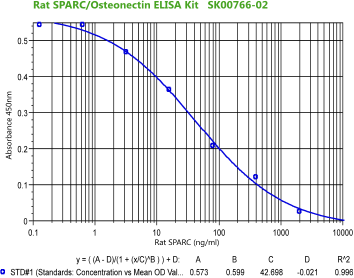
Rat/Mouse
SPARC (Osteonectin) ELISA
Code No.: SK00766-02
Size: 96 T
Standard Range: 0.128-2000 ng/ml
Dynamic Range: 0.64- 2000 ng/ml
Sensitivity: 0.128 ng/ml
Sample Type: serum, EDTA plasma
Sample requires: 120 µl, 50 µl per well
Dilution Factor: 2~4 (Optimal dilutions should be determined by each
laboratory for each application)
IntraCV: 4-6%
InterCV: 8-10%
Protocol: PDF
|
|

|
Rat
SPARC(Osteonectin) ELISA
Code No.: SK00766-09
Size: 96 T
Standard Range:0.312 -20 ng/ml
Sensitivity:0.05 ng/ml
Sample Type: serum, EDTA plasma
Sample requres: 120 µl, 50 µl per well
Dilution Factor: Optimal dilutions should be determined by each
laboratory for each application
IntraCV: 4-6%
InterCV: 8-10%
Protocol: PDF
|
|
|
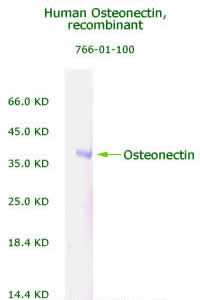
Human
Osteonectin/SPARC Recombinant
Code No.: 00766-01-100
Size: 100 µg
Protein ID: P09486
Gene ID: 6678
MW:36 KD
Tag: His Tag on N-Terminus
Expressed: E. Coli
Purity: 95%
Data Sheet: PDF
|
|
|
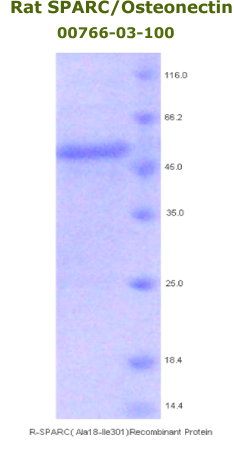
Rat
Osteonectin/SPARC Recombinant
Code No.: 00766-03-100
Size: 100 µg
Protein ID: P16975
Gene ID: 24791
MW:50 KD
Tag: His Tag on N-Terminus
Expressed: E. Coli
Purity: 95%
Data Sheet: PDF
|
|
|
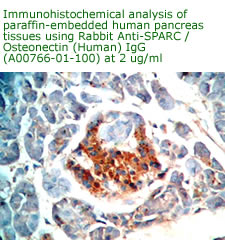
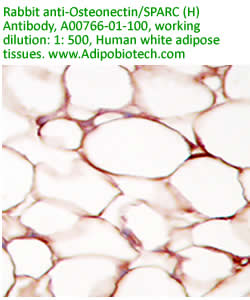
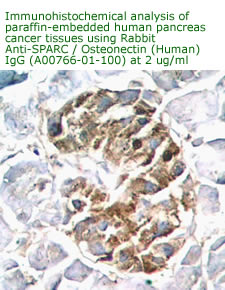
Anti
Human Osteonectin/SPARC IgG
Code No.: A00766-01-100
Size: 100 µg
Host: Rabbit
Antigen: human SPARC Rec.
Ab Type: Polyclonal IgG
Purification: Protein A
Applications: E, IHC
Working Dilution: 2 µg/ml)
Data Sheet: PDF
|
|
|
|
|
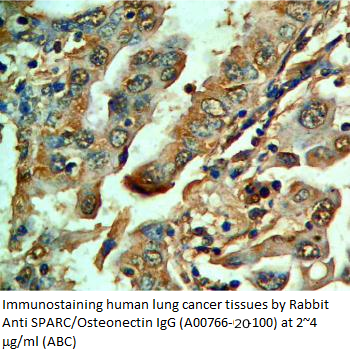 |
Anti Rat
Osteonectin/SPARC IgG Code No.: A00766-20-100 Size: 100 µg Host: Rabbit Antigen: rat SPARC Rec. Ab Type: Polyclonal IgG Purification: Protein A Applications: WB, E, IHC Working Dilution: WB (0.25-0.5 µg/ml)
Data Sheet: PDF
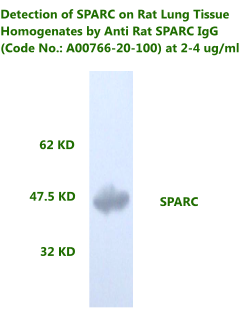
|
|

|
|
Name
|
Catalog Number
|
Size
|
Price (USD)
|
|
ELISA Kit
|
|
SPARC/Osteonectin (Human) ELISA Kit
|
SK00766-06
|
96T
|
|
|
SPARC/Osteonectin (Human) ELISA Kit
|
SK00766-01
|
96T
|
|
|
Rat/Mouse SPARC ELISA Kit
|
SK00766-02
|
96T
|
|
|
Rat SPARC ELISA Kit
|
SK00766-09
|
96T
|
|
|
Recombinant
|
|
SPARC/Osteonectin (Human) Recombinant
|
00766-01-50
|
50µg
|
|
|
SPARC/Osteonectin (Human) Recombinant
|
00766-01-100
|
100µg
|
|
|
SPARC/Osteonectin (Human) Recombinant
|
00766-01-1000
|
1mg
|
|
|
SPARC/Osteonectin (Human) Recombinant, 293 cell derived
|
00766-06-10
|
10µg
|
|
|
SPARC/OSteonectin (Human) Recombinant, 293 cell derived
|
00766-06-50
|
50µg
|
|
|
SPARC/Osteonectin (Mouse) Recombinant
|
00766-07-10
|
10µg
|
|
|
SPARC/Osteonectin (Mouse) Recombinant, Biotinylated
|
00766-07-50
|
50µg
|
|
|
SPARC/Osteonectin (Rat) Recombinant
|
00766-03-100
|
50µg
|
| Antibody
|
|
Anti SPARC/Osteonectin (Human) lgG Antibody
|
A00766-01-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) lgG Antibody, Cy3 conjugated
|
A00766-01-50C3
|
50µg
|
| Anti SPARC/Osteonectin (Human) lgG Antibody, Cy5 conjugated
|
A00766-01-50C5
|
50µg
|
| Anti SPARC/Osteonectin (Human) lgG Antibody, FAM conjugated
|
A00766-01-50F
|
50µg
|
| Anti SPARC/Osteonectin (Human) lgG Antibody, Rhodamine B conjugated
|
A00766-01-50RH
|
100µg
|
| Anti SPARC/Osteonectin (Human) Antibody, Biotinylated conjugated
|
A00766-12-50B
|
50µg
|
|
|
Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-03-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-04-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal lgG Antibody, Biotinylated
|
A00766-04-50B
|
50µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal lgG Antibody, Cy3 conjugated
|
A00766-04-50C3
|
50µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-05-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-08-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-09-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-10-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-11-100
|
100µg
|
| Anti SPARC/Osteonectin (Human) Monoclonal Antibody
|
A00766-16-100
|
100µg
|
| Anti Rat SPARC/Osteonectin lgG Antibody
|
A00766-20-100
|
100µg
|
|
|
Anti SPARC/Osteonectin (Rat) Monoclonal Antibody
|
A00766-21-100
|
100µg
|
| Anti SPARC/Osteonectin (Rat) Monoclonal Antibody
|
A00766-22-100
|
100µg
|
|
|
References
1: Nie J,et al. Inactivation of SPARC enhances high-fat diet-induced
obesity in mice. Connect Tissue Res. 2010 Jul 8. [Epub ahead of print]
2: Witkiewicz AK, et al. Stromal CD10 and SPARC expression in ductal
carcinoma in situ (DCIS) patients predicts disease recurrence. Cancer Biol
Ther. 2010 Aug 21;10(4). [Epub ahead of print]
3: Trombetta JM, Bradshaw AD. SPARC/Osteonectin Functions to Maintain
Homeostasis of the Collagenous Extracellular Matrix in the Periodontal
Ligament. J Histochem Cytochem. 2010 Jun
21. [Epub ahead of print]
4: Liang JF, et al. Relationship and prognostic significance of SPARC
and VEGF protein expression in colon cancer. J Exp Clin Cancer Res. 2010 Jun
16;29:71. PubMed PMID: 20565704;
5: Miyoshi K, et al. SPARC mRNA expression as a prognostic marker for
pancreatic adenocarcinoma patients. Anticancer Res. 2010 Mar;30(3):867-71.
6: Nagai MA, et al. Prognostic value of NDRG1 and SPARC protein
expression in breast cancer patients. Breast Cancer Res Treat. 2010 Apr 6.
[Epub ahead of print]
7: Hsiao YH, et al. SPARC (osteonectin) in breast tumors of different histologic types
and its role in the outcome of invasive ductal carcinoma. Breast J. 2010
May-Jun;16(3):305-8. Epub 2010 Feb 23.
8: Kos K, Wilding JP. SPARC: a key player in the pathologies associated
with obesity and diabetes. Nat Rev Endocrinol. 2010 Apr;6(4):225-35. Epub
2010 Mar 2. Review.
9: Capper D, et al. Secreted protein, acidic and rich in cysteine
(SPARC) expression in astrocytic tumour cells negatively correlates with
proliferation, while vascular SPARC expression is associated with patient
survival. Neuropathol Appl Neurobiol. 2010
Apr;36(3):183-97. Epub 2010 Feb 4.
10: Shen LC, et al. Expression of osteonectin/secreted protein acidic
and rich in cysteine and matrix metalloproteinases in ameloblastoma. J Oral
Pathol Med. 2010 Mar;39(3):242-9. Epub 2010 Jan 11.
11: Inoue M, et al. Identification of SPARC as a candidate target
antigen for immunotherapy of
various cancers. Int J Cancer. 2010 Sep 1;127(6):1393-403.
|
|














